PerkinElmer의 '극성물질 분석에서 C18 AQ 컬럼과 C18 AQ plus 컬럼의 선택성 비교'를 이용한 응용자료는 한국퍼킨엘머에서 제공하였으며 주요 내용은 다음과 같다.
Introduction
In recent years there has been an increase in interest regarding ‘AQ’ columns due to their ability to retain polar analytes reproducibly under high aqueous conditions and with no phase collapse as a traditional C18 column would exhibit.
There are two general approaches to the bonded phase chemistry of AQ columns. The first is to employ a polar or hydrophilic end-capping. The second method is to incorporate polar-embedded groups within the alkyl chains. These two methods provide a high degree of polar character to the final alkyl bonded phase, allowing full interaction with the alkyl chains upon wetting the silica surface with water.
Additionally, the added polar functionality introduces a secondary separation mechanism(Dipole-dipole interactions) to facilitate alternative selectivity for polar compounds.
This application brief describes the use of Quasar™ C18, AQ and AQ Plus columns for the analysis of polar compounds, outlining the benefits of stationary phases offering alternative selectivity.
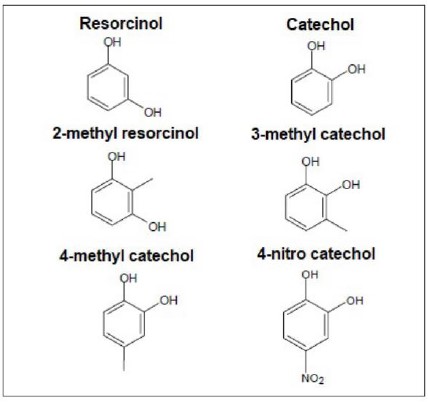
Figure 1 shows the compounds used in this study(Catechol, resorcinol and various derivatives of these compounds).
Figure 1. Chemical structure of resorcinol, catechol and some of their various derivatives.
Experimental Conditions
Method Parameters
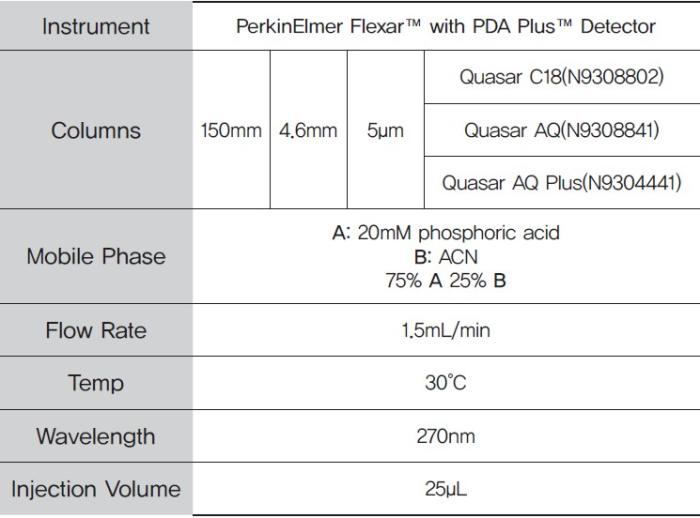
HPLC method parameters are shown in Table 1.
Table 1. HPLC method parameters.
Solvents and Samples
All solvents were HPLC grade and samples were filtered using a 0.22μm PTFE filter, P/N 02542924.
Individual stock standard solutions(1mg/mL) were prepared in 20mM phosphoric acid and sonicated. From these solutions, a combined working standard solution(0.05mg/mL) was prepared using 20 mM phosphoric acid as the diluent.
Results and Discussion
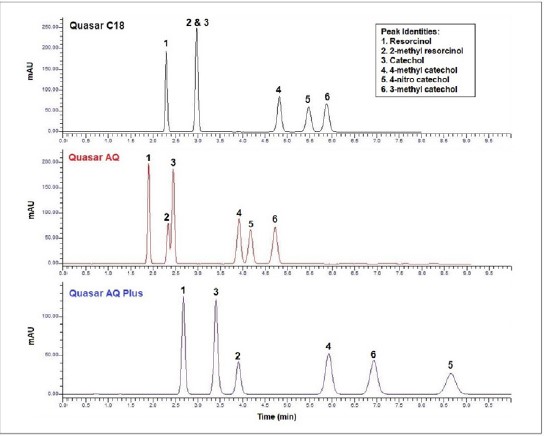
The analysis of resorcinol, catechol and various derivatives is shown in Figure 2 using the Quasar C18, AQ and AQ Plus columns. Using the Quasar C18 column, compounds two and three co-elute and the remaining compounds are baseline resolved(Rs > 1.5). This C18 phase has C18 alkyl ligands bonded to the surface of the silica, with hydrophobic end-capping. Therefore, the only interaction between analyte and stationary phase is hydrophobic interaction.
Using the same experimental conditions on a Quasar AQ column allows the benefits of changes in selectivity to be realized.
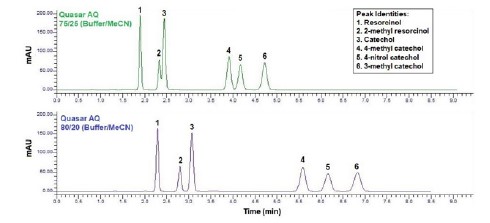
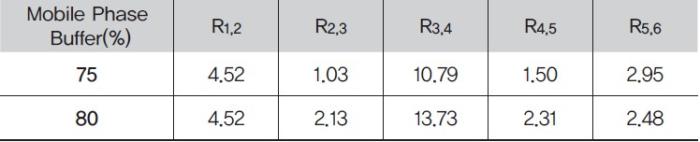
The Quasar AQ phase utilizes polar end-capping. The addition of a polar end-cap provides a higher degree of polar character to the final alkyl bonded phase, facilitating dipole-dipole interactions between the analyte and the stationary phase. The introduction of a secondary separation mechanism can lead to different retention behavior for polar analytes over a standard alkyl phase. In this case, compounds two and three begin to separate but are not baseline resolved(Rs = 1.03). The remaining peaks are baseline resolved and demonstrate the same elution order as the Quasar C18. To further increase resolution and improve retention, the aqueous content of the mobile phase can be increased(Figure 3). By simply adding 5% more aqueous content, the resolution between compounds two and three is greatly enhanced, achieving more than baseline resolution(Rs). Additionally, the resolution between compounds four and five has increased(Table 2).
Using an AQ Plus column, under the same conditions as the C18 and AQ columns(Figure 2), shows a large change in selectivity and baseline resolution between all compounds. The elution order has altered slightly, and greater retention has been achieved. The AQ Plus has proprietary end-capping and bonding with polar functionality to facilitate this change in selectivity. It utilizes a mixture of C18 alkyl ligands and polar embedded alkyl chain ligands.
It is evident that switching from a C18 column to another column such as an AQ or an AQ Plus can provide alternative selectivity for more polar compounds. Although these phases offer the possibility of strong hydrophobic interaction, the added polar functionalities introduce additional modes of interaction. The AQ and AQ Plus columns also have the added benefit of being able to robustly retain polar analytes under 100 % aqueous conditions with no phase collapse. A traditional C18 column, on the other hand, would not withstand these conditions. The column would de-wet and undergo hydrophobic collapse.
Figure 2. Analysis of resorcinol, catechol and derivatives using a Quasar C18, AQ and AQ Plus column under the conditions in Table 1.
Figure 3. Analysis of resorcinol, catechol and derivatives using the Quasar AQ with 75 % and 80 % buffer conditions.
Table 2. Resolution between peaks using the Quasar AQ column under 75% and 80% buffer conditions.
Conclusion
•Initial screening of various column stationary phase chemistries is often the most powerful approach in method development to observe changes in selectivity.
•The Quasar AQ and AQ Plus columns have shown to provide alternative selectivity for polar compounds(catechols and resorcinols), in comparison with a Quasar C18 column, due to the added polar functionality of these phases.
•Excellent peak shape was obtained due to Quasar’s ultra-high purity silica and optimized ligand bonding technology.
PerkinElmer의 '극성물질 분석에서 C18 AQ 컬럼과 C18 AQ plus 컬럼의 선택성 비교'에 대한 궁금한 내용은 본 원고자료를 제공한 한국퍼킨엘머를 통하여 확인할 수 있다.
Reference(참고문헌): https://www.hplc.eu/Downloads/AQ_Columns01.pdf, Date Accessed: July 2020.
Model Name(모델명): Quasar™ C18, AQ and AQ Plus columns
The Person in Charge(담당자): Wangyu kim
Maker(제조사): PerkinElmer
Country of Origin(원산지): UK
e-mail: Wang-yu.kim@perkinelmer.com
Data Services(자료제공): PerkinElmer Korea
<이 기사는 사이언스21 매거진 2020년 12월호에 게재 되었습니다.>