Gilson Inc의 'GX-271 Oligo Purification System: Streamlined Purification of Assay‑Ready Oligonucleotides by Automated HPLC'을 이용한 응용자료는 한국분석기기(주)에서 제공하였으며 주요 내용은 다음과 같다.
APPLICATION BENEFITS
Custom manufactured oligonucleotides require additional purification steps after synthesis to separate the full‑length products from contaminants such as truncated sequences, base protecting groups, and other impurities. Efficient purification of oligonucleotides, of different lengths and at different production scales, presents a manufacturing challenge.
SOLUTIONS
The GX-271 Oligo Purification System, configured with automated preparative and analytical HPLC capabilities, can be used to isolate, analyze, and desalt reagent-grade oligonucleotides in one continuous run. On-board fraction analysis during runs enables on‑the-fly sample addition resulting in no downtime on the instrument. Built-in error handling conditions at various stages of the process prevent sample loss and assure full walk‑away capabilities.
ABSTRACT
Figure 1. GX-271 Oligo Purification System
|
|
Oligonucleotides must be of high purity when used in molecular biology applications, but efficient purification over a large range of production scales is challenging. The Gilson GX-271 Oligonucleotide Purification System(Figure 1) provides a versatile solution for continuous separation, analysis, and desalting of oligonucleotides.
INTRODUCTION
Oligonucleotides are crucial components in many molecular biology techniques, such as qPCR, next generation sequencing(NGS), and microarray analysis and also have applications as therapeutics. These custom manufactured compounds require additional purification steps after large scale synthesis. The full-length products must be separated from truncated sequences, inhibiting salts, and base protecting groups. Efficient purification of oligonucleotides, of different lengths and at different production scales, presents a manufacturing challenge.
To overcome these challenges, Gilson has developed a single liquid handling platform configured with automated preparative and analytical HPLC capabilities that can be used to isolate, analyze, and desalt reagent-grade oligonucleotides in one continuous run.
MATERIALS AND METHODS
All solvents used for chromatography were HPLC grade. Gilson TRILUTION® LC software was used to control instrumentation during the purification process.
Crude synthetic oligonucleotides were injected onto a preparative ion-exchange column. Small samples of selected column fractions were reinjected to confirm identity. Fractions with established identities were desalted with a size-exclusion desalting column.
Samples and Reagents
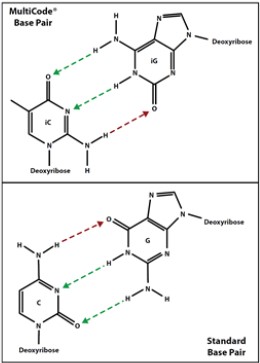
Crude synthetic oligonucleotides were used as-is following laboratory synthesis.(Figure 2)
Figure 2. Luminex MultiCode® Technology
|
|
INSTRUMENTATION
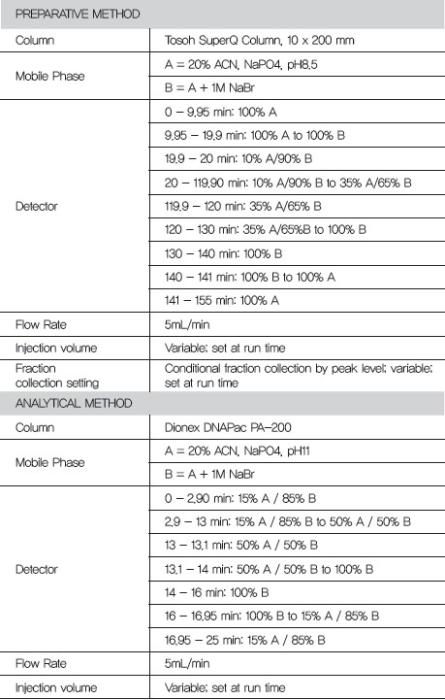
Chromatography Methods
RESULTS AND DISCUSSION
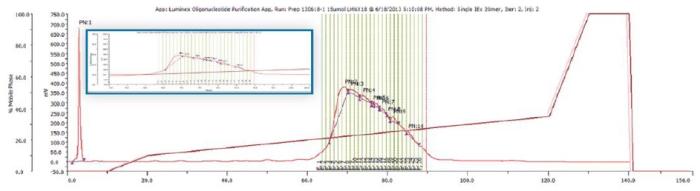
The GX-271 Oligo Purification System is configured with both analytical and preparative injection ports, selection valves to accommodate different size chromatography columns, and with the ability to vary the sample load volume. To illustrate the capabilities of this system, we will walk through an example purification. Crude synthetic oligonucleotides (8 - 40 mers) were first injected onto a preparative reverse phase or ion exchange HPLC column and peaks were collected via conditional fraction collection.
That is, fractions were automatically collected by the instrumentation when the Abs260 slope met the criteria established in the method. This is shown in Figure 3. The instrumentation then removed small samples from the tubes that contained fractions and injected them directly onto a reverse phase or anion exchange analytical column to confirm oligonucleotide size and purity.(Figure 4)
Figure 3. Pilot Scale Purification of 20 mer ligonucleotide.
Figure4. Analytic Verification of 20 mer Oligonucleotide
Figure 5. Phosphate Buffer Gradient Monitoring with the VERITY® 1810 Conductivity and pH Monitor
| |
| |
|
|
In the final purification step, fractions that passed the previous size and purity test were automatically injected onto a size-exclusion desalting column, achieving >90% oligonucleotide purity. Effective salt removal was confirmed by in-line UV and conductivity monitoring.
Modular and flexible programming in the TRILUTION LC software facilitated seamless exchange of preparative and analytic methods without manual intervention. On-board fraction analysis during runs enabled the loading of sample lists for subsequent runs, resulting in no downtime on the instrument. Additionally, built-in error handling conditions at various stages of the process prevented sample loss and allowed for fully automated, unattended operation. In the event of an error, the run can be stopped entirely or another method can be executed to enable sample recovery.
The versatility of the GX-271 Oligo Purification System permitted continuous sample injection, collection, reinjection, fraction pooling, and desalting on an automated platform, providing bulk purified oligonucleotides as the end product for use in molecular biology
applications.
The GX-271 Oligo Purification System makes use of the VERITY® 1810 Conductivity and pH Monitor to observe salt concentrations. This state-ofthe-art instrument is capable of monitoring salt concentrations over a large dynamic range with a rapid response time in biological purifications, and is also equipped with pH monitoring capabilities.
Figure 5 shows an example of phosphate buffer gradient data collected with the VERITY 1810 Conductivity and pH Monitor.
REFERENCES
1. Bonilla, Jose V., and G. Susan Srivasta, eds. Handbook of Analysis of Oligonucleotides and Related Products. CRC Press, 2011.
ACKNOWLEDGMENTS
The oligonucleotide purification work was carried out by Luminex; Madison, WI. The VERITY® 1810 Conductivity and pH Monitor work was carried out by Gilson, Inc.; Middleton, WI.
CONCLUSIONS
The challenge of purifying a range of oligonucleotides(8 - 40 mers) in various injection volumes (up to 1.5L) on different Chromatography columns is overcome by the modularity of the Gilson instrumentation and the flexibility of the TRILUTION® LC software.
The versatility of the GX-271 Oligo Purification System and TRILUTION® LC software permit easy switching between hardware components and columns accommodating sequential preparative HPLC, analytical HPLC, and large-scale desalting functionalities on
one system.
On-board fraction analysis during runs enables on‑the-fly sample addition resulting in no down-time on the instrument.
Built-in error handling conditions at various stages of the process prevent sample loss and assure full walk‑away capabilities.
Continuous sample injection, collection, reinjection, fraction pooling, and desalting on a single automated platform improves manufacturing efficiencies of oligonucleotides used in molecular biology applications.
TRADEMARKS
All product and company names are trademarks™ or registered® trademarks of their respective holders. Use of the trademark(s) in this document does not imply any affiliation with or endorsements by the trademark holder(s).
MultiCode® is a registered trademark of Luminex.
Gilson Inc의 'GX-271 Oligo Purification System: Streamlined Purification of Assay‑Ready Oligonucleotides by Automated HPLC'에 대한 궁금한 내용은 본 원고자료를 제공한 한국분석기기(주)를 통하여 확인할 수 있다.
Reference(참고문헌): Gilson Application note 1031
Model Name(모델명): GX-271 Oligo Purification System
The Person in Charge(담당자): An Hyesook
Maker(제조사): Gilson Inc.
Country of Origin(원산지): U.S.A
e-mail: kaisco1@kaisco.co.krData Services(자료제공): Gilson Inc.
<이 기사는 사이언스21 매거진 2020년 7월호에 게재 되었습니다.> |