Bruker의 'Fast and Reliable Carbon and Sulfur Determination in Limestone, Dolomite and Lime'에 관한 응용자료의 주요 내용은 다음과 같다.
Introduction
Limestone and Dolomite are sed imenta ry rocks w ith a tremendous variety of applications in a wide variety of fields. Limestone is used as construction raw material, aggregate, fluxing agent, acid neutralizing agent, and an animal feed filler. When calcined, burnt lime(CaO) is created, that is more effective in acid�neutralization and a construction material. Dolomite is harder than limestone, making it a superior construction material.
Quality- and Process-Control require the fast and accurate analysis of the elemental composition in these raw materials. This Lab Report shows the simplicity, speed and reliability of carbon and sulfur determination by the G4 ICARUS Series 2 using a high�frequency(HF) induction furnace.
Measuring Principle
The combustion takes place under a flow of oxygen within a sealed HF induction furnace. While the sample, with addition of a metallic accelerator, is heated by induction, it combusts at uploaderatures
exceeding 2000 °C. Sulfur compounds are oxidized to SO2 and carbon to CO2. Total carbon and sulfur are calculated relative to the amount of these combustion gases, which are quantified by selective detection systems.
Combustion Without Compromises: G4 ICARUS!
With its powerful HF-furnace equipped with ZoneProtect™, its unique vacuum-free automatic cleaning system, HighSense™ detectors and electronic flow- and pressure-control, the G4 ICARUS Series 2 is a smart addition for every industrial user who depends on a reliable instrument even under harsh conditions. The G4 ICARUS Series 2 is capable to measure both carbon and sulfur in cements and related materials with high precision in typically below one minute.
Sample Preparation
For optimal precision, the sample should be grinded or crushed to a uniform powder prior to analysis. If necessary, it should to be dried for at least 1 h at 110°C. No further sample preparation is necessary.
Method Parameters
•Purge time: 5s
•Start delay: 5s
•Baseline check before analysis: 5s
•Analysis time 1: 35s(power Level: 4)
•Analysis time 2: 25s(power level: 0)
•Baseline check after analysis: 5s
•Crucible cool time: 10s
•Sample mass: ~0.2g(Weighed to 0.1mg)
•Accelerators:
- Tungsten/Tin: ~1.5g
- High purity iron chips: ~0.7g
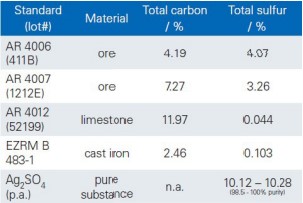
Calibration
The calibration of the analyzer is performed by means of reference materials with certified concentrations of carbon and sulfur. The analysis software supports single point calibration with one CRM and multi point calibration with multiple CRMs.
Procedure
I. Determination of the blank value
Run a minimum of 3 analysis of the blank value by adding the described amount of accelerators into a preheated1) crucible and analyze.
1) For optimal precision, ceramic crucibles shall be pre-heated in a muffle furnace at ≥1250 °C for a minimum of 15 min or ≥1000 °C for a minimum of 2 hours. To avoid contamination, crucibles must be handled with clean tongs and transferred to a desiccator for storage.
II. Measuring reference materials
• Choose CRMs for calibration and define them in the analysis software with designation and the certified concentrations.
• Weigh in an appropriate amount of reference material into a preheated1) crucible, and transfer the exact mass into the analysis software. Cover the material with the described amount of accelerator and analyze.
• Repeat step 2 a minimum of three times for each reference material(or sample weight) used.
Calibrate the analyzer with the blank values recorded under I. and the results obtained with reference materials
III. Sample measurement
• Weigh ~0.2 g of sample into a preheated1) crucible and transfer the exact sample mass to the analysis software. Cover the sample with the described amount of accelerator and analyze.
• Repeat step 1 until an appropriate number of repetitions is obtained.
Calibration Example
Typical concentration levels for total carbon and total sulfur in Limestone and Dolomite are in the range of Carbon: 10-15% and Sulfur: 0.02-0.4%, whereas the typical contents in burnt lime are
Carbon: 0.1-2% and Sulfur: 0.01-0.25%.
The typical concentration range is useful information to consider when selecting appropriate reference material for calibration.
Although all detectors are linearized and an extrapolation of the calibration curve is possible, it is good laboratory practice that all samples fall within the calibrated range. For this Lab Report, the calibration and method is identical to that used for cement in Lab Report CS/ONH 020.
The resulting calibration for carbon and sulfur yield a R2=0.9999 which proves the excellent linearity, accuracy and reproducibility of the G4 ICARUS Series 2 detection system and the entire method.
Table 1: Used reference materials
Results
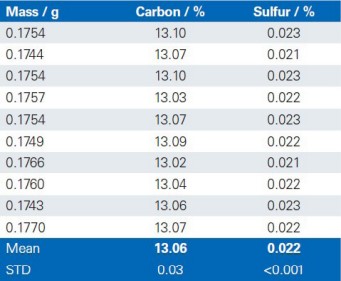
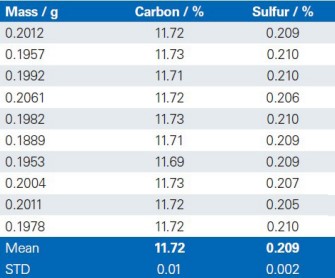
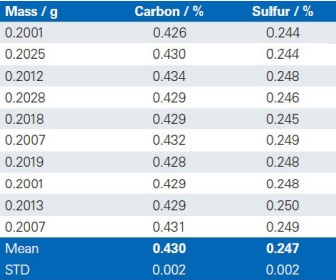
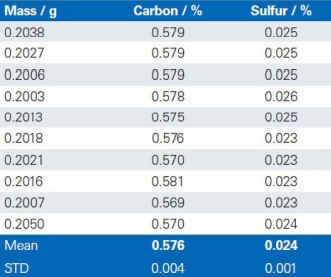
The reproducibility of the G4 ICARUS Series 2 and the method outlined is demonstrated by a series of 10 repetitive measurements of real production samples.
Dolomite
Limestone
Lime 1
Lime 2
Summary
The combination of HighSense™ detection systems with precise electronic flow control in the G4 ICARUS Series 2 delivers excellent and long-time stable analytical performance. The powerful HF�furnace equipped with the industry-leading ZoneProtect™ and its unique automatic cleaner ensures lowest cost of ownership, high availability with little maintenance. The sample preparation for this method is straightforward and does not require any expert knowledge. The analytical performance in combination with the speed of analysis and its ease of use makes the G4 ICARUS Series 2 the ideal choice for the quality control of limestone, dolomite, burnt lime and related products.
Bruker의 'G4 ICARUS'에 관한 궁금한 내용은 본 원고자료를 제공한 DKSH 코리아(주)를 통하여 확인할 수 있다.
Reference(참고문헌): Bruker application note
Model Name(모델명): G4 ICARUS
The Person in Charge(담당자): Lee Jaeyun
Maker(제조사): Bruker
Country of Origin(원산지): Germany
Mail inquiry: dksh.info.tec@dksh.com
Data Services(자료제공): DKSH Korea
<이 기사는 사이언스21 매거진 2022년 4월호에 게재 되었습니다.>